Cortisol vs. Your Other Hormones: The Battle Explained
Imagine your hormonal system as a sophisticated orchestra, with each hormone playing its unique part to create the symphony of your health. Now picture cortisol as an overzealous conductor who, when stressed, begins drowning out the other musicians until the beautiful harmony becomes discordant chaos. This is exactly what happens in your body when cortisol—your primary stress hormone—goes from helpful ally to hormonal dictator.
Understanding this internal battle isn't just academic curiosity; it's the key to unlocking why you might feel tired but wired, hungry but never satisfied, or simultaneously anxious and depressed. When cortisol declares war on your other hormones, every system in your body feels the impact.
Meet the Players: Your Hormonal Cast
Before diving into the conflict, let's understand who's involved in this complex biological drama. Your endocrine system operates like a delicately balanced ecosystem, where each hormone has specific roles and relationships with others.
Cortisol serves as your body's alarm system and emergency response coordinator. In healthy amounts and timing, it helps you wake up in the morning, respond to challenges, and maintain blood sugar stability. Think of cortisol as your internal bodyguard—essential for protection, but problematic when it never stands down.
Insulin manages your energy storage and blood sugar levels, working closely with cortisol in ways that can either support metabolic health or create dysfunction. When cortisol chronically elevates, insulin sensitivity decreases, leading to energy storage problems and increased hunger.
Thyroid hormones (T3 and T4) control your metabolic rate, energy production, and cellular function throughout your body. They're like the engine of your car—when they're functioning optimally, everything runs smoothly, but when cortisol interferes, the engine starts sputtering.
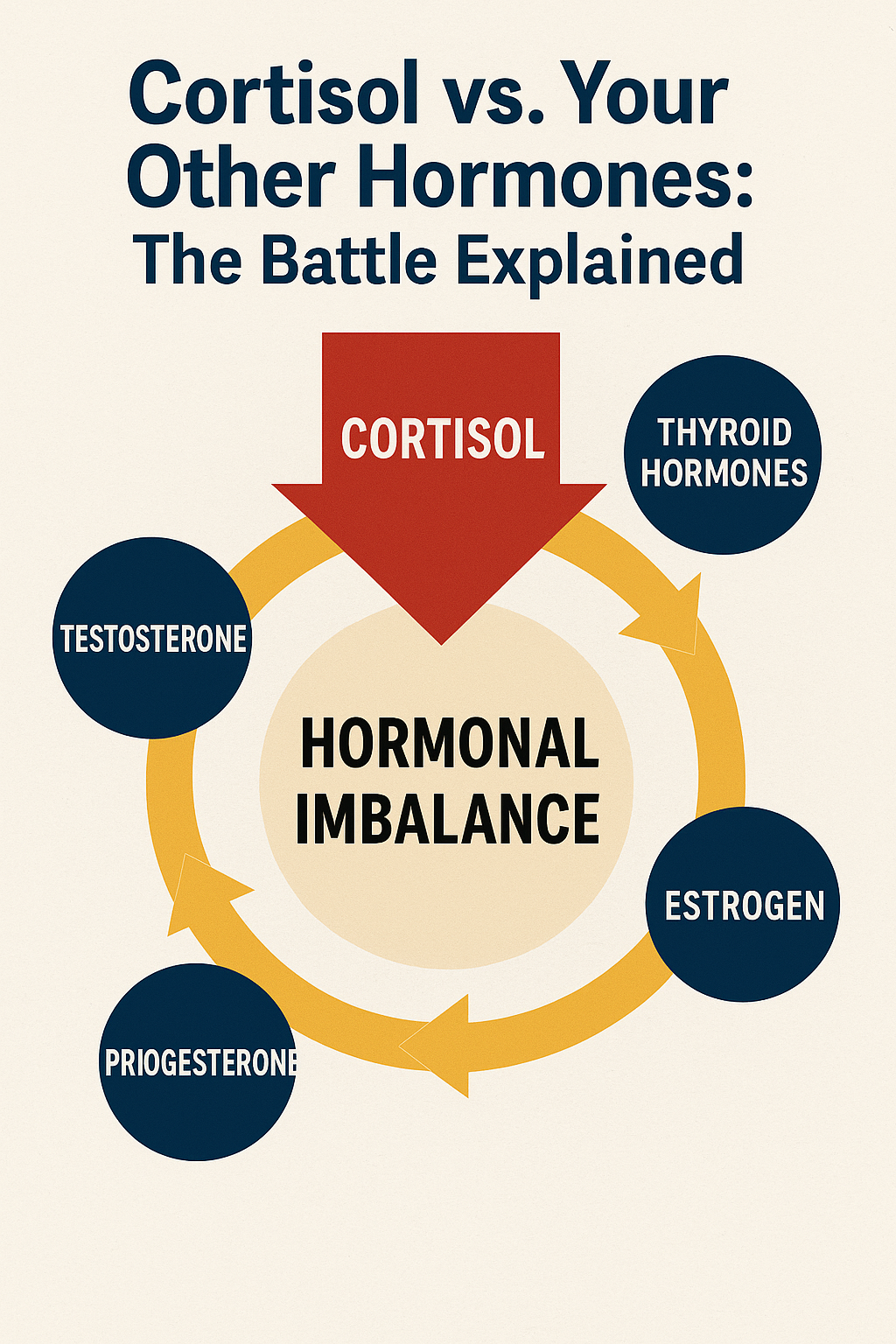
Reproductive hormones (estrogen, progesterone, and testosterone) govern not just reproduction but also mood, energy, muscle mass, bone density, and cognitive function. These hormones often bear the brunt of cortisol's dominance, as your body prioritizes immediate survival over long-term reproductive health.
Sleep hormones like melatonin regulate your circadian rhythms and recovery processes. When cortisol overstays its welcome, it directly opposes melatonin production, creating the tired-but-wired sensation many people experience.
The Hierarchy of Survival: Why Cortisol Always Wins
Your body operates on a biological hierarchy where immediate survival trumps everything else. This ancient programming served our ancestors well when facing genuine life-or-death situations, but it becomes problematic when your nervous system can't distinguish between a charging saber-tooth tiger and a demanding boss.
When your brain perceives threat—whether it's a looming deadline, relationship conflict, or financial stress—it triggers the hypothalamic-pituitary-adrenal (HPA) axis. This cascade results in cortisol release, which mobilizes resources for immediate action. The problem arises when this system never turns off.
Cortisol steals resources from other hormone production through a process called "pregnenolone steal." Pregnenolone is the mother hormone from which many other hormones are made. When cortisol demand is high, your body diverts pregnenolone toward cortisol production, leaving fewer raw materials for sex hormones, leading to what's often called "adrenal fatigue" or HPA axis dysfunction.
This resource allocation explains why chronic stress often leads to low libido, irregular periods, mood swings, and fertility issues. Your body is essentially saying, "We don't have time for reproduction right now—we're fighting for survival."
Round 1: Cortisol vs. Insulin (The Energy Storage Battle)
The relationship between cortisol and insulin resembles a dysfunctional partnership where both are trying to manage your energy, but their methods increasingly conflict as stress becomes chronic.
Cortisol's job includes maintaining blood sugar levels during stress by promoting gluconeogenesis—the production of glucose from non-carbohydrate sources. This ensures your brain and muscles have immediate fuel for the fight-or-flight response. In acute situations, this is brilliant biological engineering.
Insulin's role is to help cells absorb glucose from your bloodstream and store excess energy for later use. Under normal circumstances, cortisol and insulin work together harmoniously, with cortisol providing energy during stress and insulin managing storage during calm periods.
The conflict begins when cortisol remains chronically elevated. High cortisol promotes insulin resistance, meaning your cells become less responsive to insulin's signals. Your pancreas responds by producing more insulin, creating a cascade of metabolic dysfunction.
This battle manifests as energy crashes after meals, difficulty losing weight despite caloric restriction, increased cravings for sugary and high-carb foods, and the tendency to store fat around the midsection. Many people trapped in this cycle feel like they're doing everything "right" nutritionally but still struggle with energy and weight management.
The collateral damage includes increased inflammation, disrupted appetite regulation, and impaired fat burning. When cortisol consistently overpowers insulin's effectiveness, your body shifts into energy storage mode, interpreting chronic stress as a signal that famine might be coming.
Round 2: Cortisol vs. Thyroid Hormones (The Metabolic Slowdown)
The thyroid-cortisol relationship illustrates how stress can systematically slow down your entire metabolic system, leaving you feeling sluggish despite being stressed and "wired."
Thyroid hormones regulate the speed of virtually every cellular process in your body. They determine how efficiently you burn calories, how quickly your heart beats, how fast your brain processes information, and how rapidly your cells repair and regenerate.
Cortisol interferes with thyroid function at multiple levels. It can suppress thyroid-stimulating hormone (TSH) production, reduce the conversion of inactive T4 to active T3, and increase thyroid-binding proteins that make thyroid hormones less available to your cells.
Additionally, chronic cortisol elevation increases inflammation and promotes the production of reverse T3, which acts like a brake on your metabolic system. Reverse T3 sits in thyroid receptors but doesn't activate them, essentially blocking your cells' ability to respond to thyroid hormones.
This explains why many people with chronic stress experience symptoms that look like hypothyroidism—fatigue, weight gain, cold intolerance, brain fog, and depression—even when their basic thyroid tests appear normal. The thyroid gland might be producing hormones, but cortisol's interference prevents those hormones from working effectively.
The recovery challenge is that standard thyroid replacement therapy often doesn't address the cortisol-driven dysfunction. Until cortisol levels normalize and inflammation decreases, thyroid hormones may remain less effective, leaving people feeling unwell despite "optimal" thyroid medication dosing.
Round 3: Cortisol vs. Reproductive Hormones (The Fertility Shutdown)
Perhaps nowhere is cortisol's dominance more evident than in its relationship with reproductive hormones. This battle has profound implications not just for fertility, but for mood, energy, cognitive function, and overall vitality.
The biological logic is straightforward: if your body believes you're in danger, reproduction becomes a luxury it can't afford. Chronic stress signals that the environment isn't safe for c


